Q.1 The water exchange rates for the complex ions follow the order
Q.2 Based on Wade’s rule, the structure-type of [B₅H₈]⁻ is
(A) closo
(B) nido
(C) arachno
(D) hypho
Ansewr:-B
Q.3 The coordination geometries around the copper ion of plastocyanin (a blue-copper protein) in oxidized and reduced form, respectively, are
(A) tetrahedral and square-planar
(B) square-planar and tetrahedral
(C) distorted tetrahedral for both
(D) ideal tetrahedral for both
Answer:-C
(A) [V(H2O)6]2+ > [Co(H2O)6]2+ > [Cr(H2O)6]3+
(B) [Cr(H2O)6]3+ > [Co(H2O)6]2+ > [V(H2O)6]2+
(C) [Co(H2O)6]2+ > [Cr(H2O)6]3+ > [V(H2O)6]2+
(D) [Co(H2O)6]2+ > [V(H2O)6]2+ > [Cr(H2O)6]3+
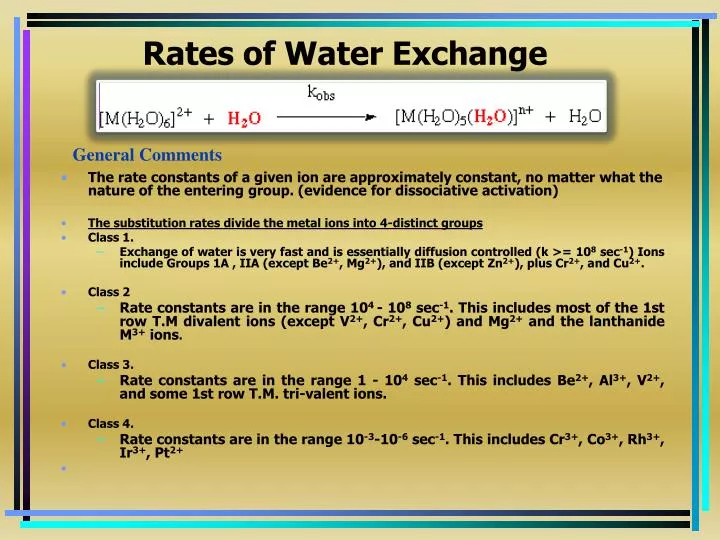

Ansewr:-D
Q.2 Based on Wade’s rule, the structure-type of [B₅H₈]⁻ is
(A) closo
(B) nido
(C) arachno
(D) hypho
Ansewr:-B
Q.3 The coordination geometries around the copper ion of plastocyanin (a blue-copper protein) in oxidized and reduced form, respectively, are
(A) tetrahedral and square-planar
(B) square-planar and tetrahedral
(C) distorted tetrahedral for both
(D) ideal tetrahedral for both
Answer:-C
No comments:
Post a Comment