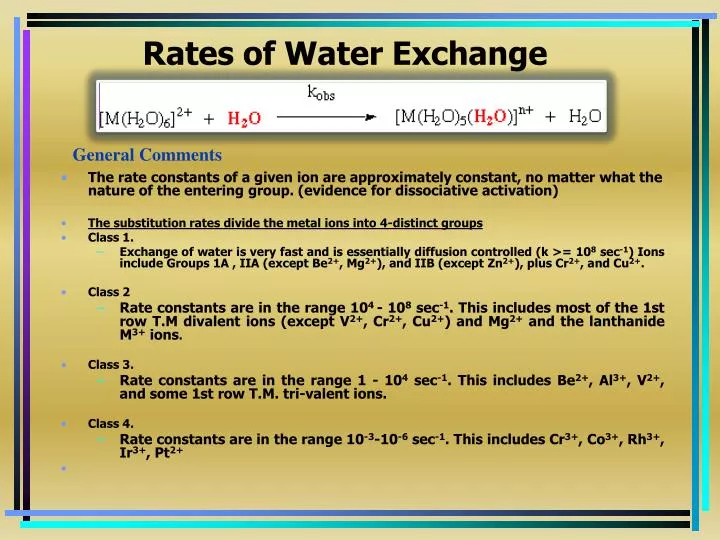
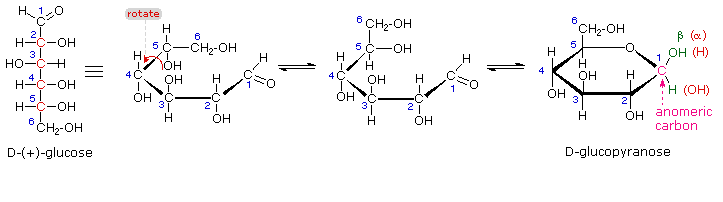
Q.1 A reaction involving two different reactants can never be
(a) Unimolecular reaction
(b) First order reaction
(c) second order reaction
(d) Bimolecular reaction

Answer (a) Unimolecular reaction
Q.2 Due to the presence of an unpaired electron, free radicals are:
(a) Chemically reactive
(b) Chemically inactive
(c) Anions
(d) Cations
Answer (a) Chemically reactive
Q.3 Lattice energy of an ionic compounds depends upon
(a) Charge on the ion only
(b) Size of the ion only
(c) Packing of ions only
(d) Charge on the ion and size of the ion
Answer (d) Charge on the ion and size of the ion
(b) 0.1 M chloroacetic acid
(c) 0.1 M fluoroacetic acid
(d) 0.1 M difluoroacetic acid
(b) Pb and Zn
(c) Ag and Au
(d) Fe and Ni
Answer (c) Ag and Au
(a) Unimolecular reaction
(b) First order reaction
(c) second order reaction
(d) Bimolecular reaction
Answer (a) Unimolecular reaction
Q.2 Due to the presence of an unpaired electron, free radicals are:
(a) Chemically reactive
(b) Chemically inactive
(c) Anions
(d) Cations
Answer (a) Chemically reactive
Q.3 Lattice energy of an ionic compounds depends upon
(a) Charge on the ion only
(b) Size of the ion only
(c) Packing of ions only
(d) Charge on the ion and size of the ion
Answer (d) Charge on the ion and size of the ion
Q.4 The highest electrical conductivity of the following aqueous solutions is of
(a) 0.1 M acetic acid(b) 0.1 M chloroacetic acid
(c) 0.1 M fluoroacetic acid
(d) 0.1 M difluoroacetic acid
Answer (d) 0.1 M difluoroacetic acid
Q.5 During the process of electrolytic refining of copper, some metals present as impurity settle as ‘anode mud’ These are
(a) Sn and Ag(b) Pb and Zn
(c) Ag and Au
(d) Fe and Ni
Answer (c) Ag and Au